Imaging Single-Cell Gene Transcription
The research theme of Mancini lab at Baylor College of Medicine, Houston, TX, USA is single-cell analysis of gene expression and they are conducting screenings of EDCs using high throughput imaging-based methods.
Endocrine disrupting chemicals (EDCs) are chemical substances that behave like hormones by binding the receptor and disrupt the original functions of hormones. They are considered to cause reproductive dysfunction and tumor and have serious effects on the human body.
The research theme of Mancini lab at Baylor College of Medicine, Houston, TX, USA is single cell analysis of gene expression and they are conducting screenings of EDCs using high throughput imaging-based methods.

Right to left: Michael A Mancini, Ph.D., Professor and Academic Core Director, Fabio Stossi, Ph.D., Associate Professor and Technical Director, and Hannah Johnson, M.S., Research Assistant
Introduction of Mancini lab
The Mancini lab has spearheaded analysis of the mechanisms of nuclear receptor gene transcription through high throughput/high content imaging. We developed one of the few existing cell engineered systems (nicknamed “PRL-array”) to simultaneously visualize and measure central steps in nuclear receptor activation of gene transcription, namely DNA binding, chromatin remodeling, coregulator recruitment, and transcriptional output (1-4). In recent years we built several biosensor cell lines for nuclear receptors including Estrogen Receptor- ∝ and -β, Androgen and Progesterone Receptor, all central transcription factors to human pathophysiology. One of the advantages of these cell lines is that they can be used for rapid testing of chemicals that interfere with these receptors, known as endocrine disrupting chemicals (EDCs), which are a continuous threat to human health and the environment.
Here we present our new model that highlights Estrogen Receptor-β (ERβ, GFP-ERβ:PRL-HeLa), a sparsely studied nuclear receptor in toxicology. We queried it against several libraries of reference compounds, lab-made and environmental complex mixtures.
Speed and high resolution are necessary to facilitate testing chemical substances
Because of the importance of rapid testing of toxicants during environmental emergencies, speed of acquisition in live cells, coupled with high resolution, is essential for fast and accurate analysis. Moreover, having the possibility of performing up to 5 fluorescent channel acquisitions greatly enhances the imaging content, opening the space for higher multi-dimensional mechanistic and/or phenotypic analysis within the same assay.
The CellVoyager CV8000 has excellent speed and resolution, and because of the large field of view and water objectives, it allows the capture of large fields of view for optimal statistical power in single-cell analytics, in both live and fixed experiments. The speed of acquisition has allowed us to cut the time of imaging each plate by more than half compared to other available instruments in our core facility, while still allowing a larger number of channels and FOVs. Now, because of the CV8000, we can query large chemical libraries and number of samples in a manageable and reproducible manner without sacrificing quality of imaging and analytics.
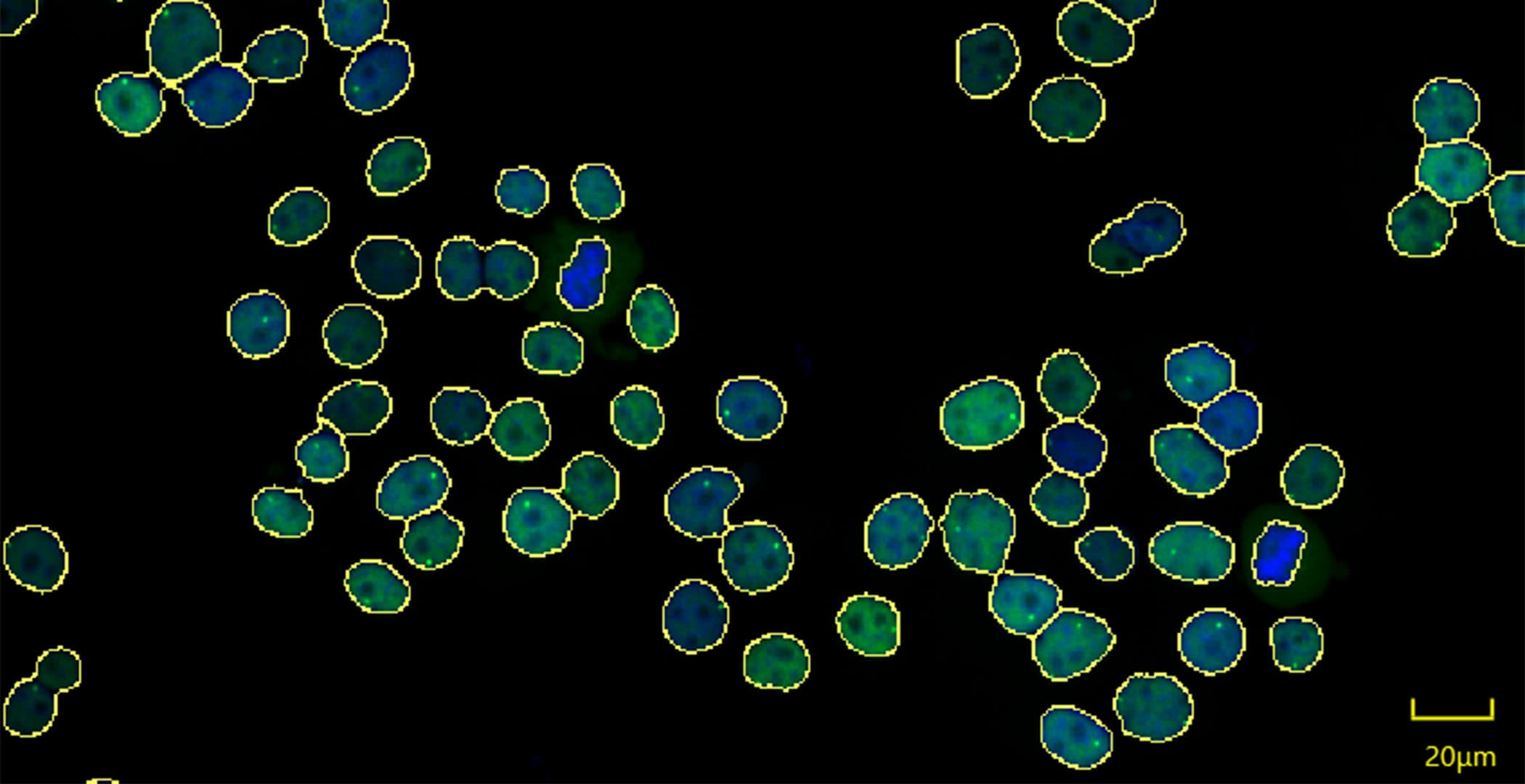
Figure 1 shows an example of the GFP-ERβ:PRL-HeLa treated with the natural hormone 17β-estradiol (E2) analyzed by CellPathfinder, which is able to easily identify the spot where gene transcription is occurring.


 Figure 1. GFP-ERβ:PRL-HeLa were treated with E2 for 2 hours and imaged on the CV8000 with the 20xLWD objective. Shown is a max projection of the GFP channel (ERβ) in green and DAPI in blue; the masks generated by CellPathfider for the nuclei (yellow outline) and the transcription locus (red outline); and, a plate map showing experimental results with the indicated feature in a 384 well plate.
Figure 1. GFP-ERβ:PRL-HeLa were treated with E2 for 2 hours and imaged on the CV8000 with the 20xLWD objective. Shown is a max projection of the GFP channel (ERβ) in green and DAPI in blue; the masks generated by CellPathfider for the nuclei (yellow outline) and the transcription locus (red outline); and, a plate map showing experimental results with the indicated feature in a 384 well plate.
Prospects for research on environmental toxicology and gene transcription
In the arena of environmental toxicology and gene transcription imaging, we envision the CV8000 to be central in all our current and future efforts, from analysis of real-life environmental mixtures to large chemical (including drug screening) and knock-out/over-expression libraries in both biosensor cell models and endogenous systems.
References
- Bolt MJ, Singh P, Obkirchner CE, Powell RT, Mancini MG, Szafran AT, Stossi F, Mancini MA. Endocrine disrupting chemicals differentially alter intranuclear dynamics and transcriptional activation of estrogen receptor-α. iScience. 2021 Oct 7;24(11):103227. doi: 10.1016/j.isci.2021.103227. PMID: 34712924; PMCID: PMC8529556.
- Sharp ZD, Mancini MG, Hinojos CA, Dai F, Berno V, Szafran AT, Smith KP, Lele TP, Ingber DE, Mancini MA. Estrogen-receptor-alpha exchange and chromatin dynamics are ligand- and domain-dependent. J Cell Sci. 2006 Oct 1;119(Pt 19):4101-16. doi: 10.1242/jcs.03161. Epub 2006 Sep 12.
- Stossi F, Bolt MJ, Ashcroft FJ, Lamerdin JE, Melnick JS, Powell RT, Dandekar RD, Mancini MG, Walker CL, Westwick JK, Mancini MA. Defining estrogenic mechanisms of bisphenol A analogs through high throughput microscopy-based contextual assays. Chem Biol. 2014 Jun 19;21(6):743-53. doi: 10.1016/j.chembiol.2014.03.013. Epub 2014 May 22. PMID: 24856822; PMCID: PMC4301571.
- Szafran AT, Stossi F, Mancini MG, Walker CL, Mancini MA. Characterizing properties of non-estrogenic substituted bisphenol analogs using high throughput microscopy and image analysis. PLoS One. 2017 Jul 13;12(7):e0180141. doi: 10.1371/journal.pone.0180141. PMID: 28704378; PMCID: PMC5509144.






