Oxygen content in blanket gas applications with Panametrics
Chemical, petrochemical, biochemical, and pharmaceutical companies, as well as research institutions, may store liquids in large tanks, have mixing chambers, or may have reaction processes where the presence of oxygen could lead to a fire or an explosion.
Application
Oxygen is measured in inert gases that blanket hydrocarbon and other organic liquids during storage, mixing, or other processes.
Customer’s Challenge
Air can leak into the vapor space above hydrocarbon liquids stored in tanks or process vessels. To remove the oxygen present due to the air leaking into the vessel, inert gases, such as nitrogen or carbon dioxide, are used to purge the vapor space above the liquids. The oxygen level must be kept below the lower explosive limit (LEL) of the hydrocarbon vapors present.
Prior to the use of thermoparamagnetic oxygen analyzers in this application, electrochemical sensors were used, often without a proper sample system, resulting in condensing hydrocarbons fouling/destroying the sensor. Frequent manual calibration to ensure the sensor responds to changes in oxygen and replacement due to liquid carry-over result in high maintenance and operating costs.
Benefits:
- Panametrics designed systems maximize uptime
- Sample system design mitigates liquid carry-over to the sensor
- XMO2 thermoparamagnetic sensor more immune to minor liquid carry-over
- XDP display/controller drives solenoid valves in the sample system for automated calibrations
Panametrics’ Solution
Continuous monitoring of the oxygen content using a thermoparamagnetic oxygen analyzer with an analyzer display package and a properly designed sample system (with solenoid valves for auto-calibration) provides maintenance-free operation. The sample system can mitigate liquid condensation reaching the sensor. The sensor design has no moving parts, thus any liquid carry-over can be easily drained. The automatic calibration triggered by the controller minimizes manual calibration.
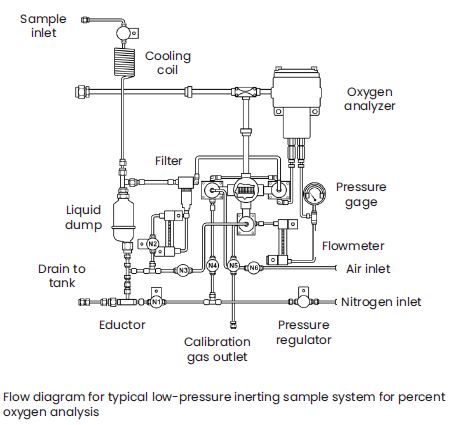
A typical installation includes a thermoparamagnetic oxygen sensor mounted in a sample system like the one shown, with a display/controller that can actuate the solenoid valves for regular auto-calibration. In most applications, ambient air is the span gas and the nitrogen from the purge system is the zero gas. Galvanic fuel cell transmitters provide a lower up-front cost alternative. They are more susceptible to liquid carry over and the sensors are a consumable spare part. The sample system design shown above would remain the same, with the galvanic transmitter replacing the thermoparamagnetic transmitter in the sample system shown.
In most applications, the appropriate location of the oxygen measurement system is at or near the top of the vessel or storage tank. The purge nitrogen source passes through the eductor in the sample system to draw the sample from the headspace. Liquids from the liquid dump, bypass filter bottom flow, and the sample are returned with the nitrogen back to the headspace in a closed-loop system, thus venting nothing to the atmosphere. Alternatively, during calibration, the span gas may be vented to atmosphere so that the system does not introduce air into the headspace, even in small amounts.
Specifications
- Application: 0 to 4% O2
- Temperature: (O2 below LEL) Balance N2 Typical range is 0 to 21% O2
- Ambient Pressure: Near 0 psig
Via Panametrics, a Baker Hughes business